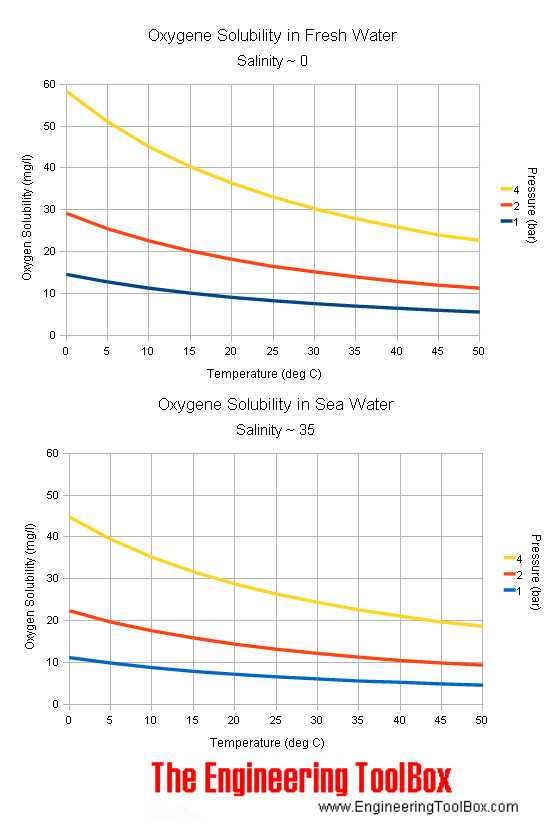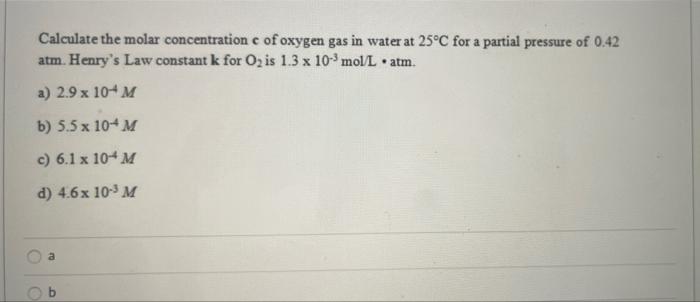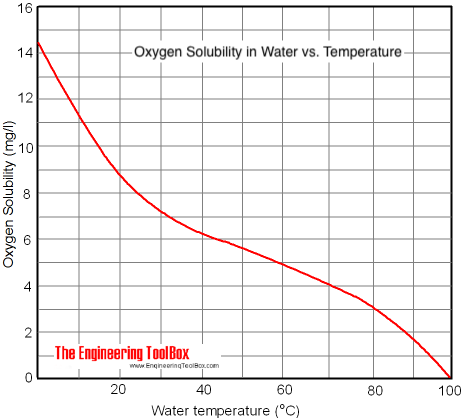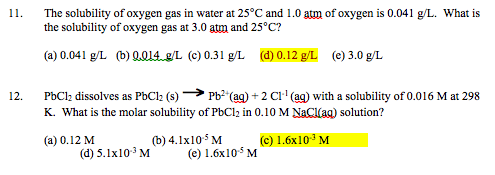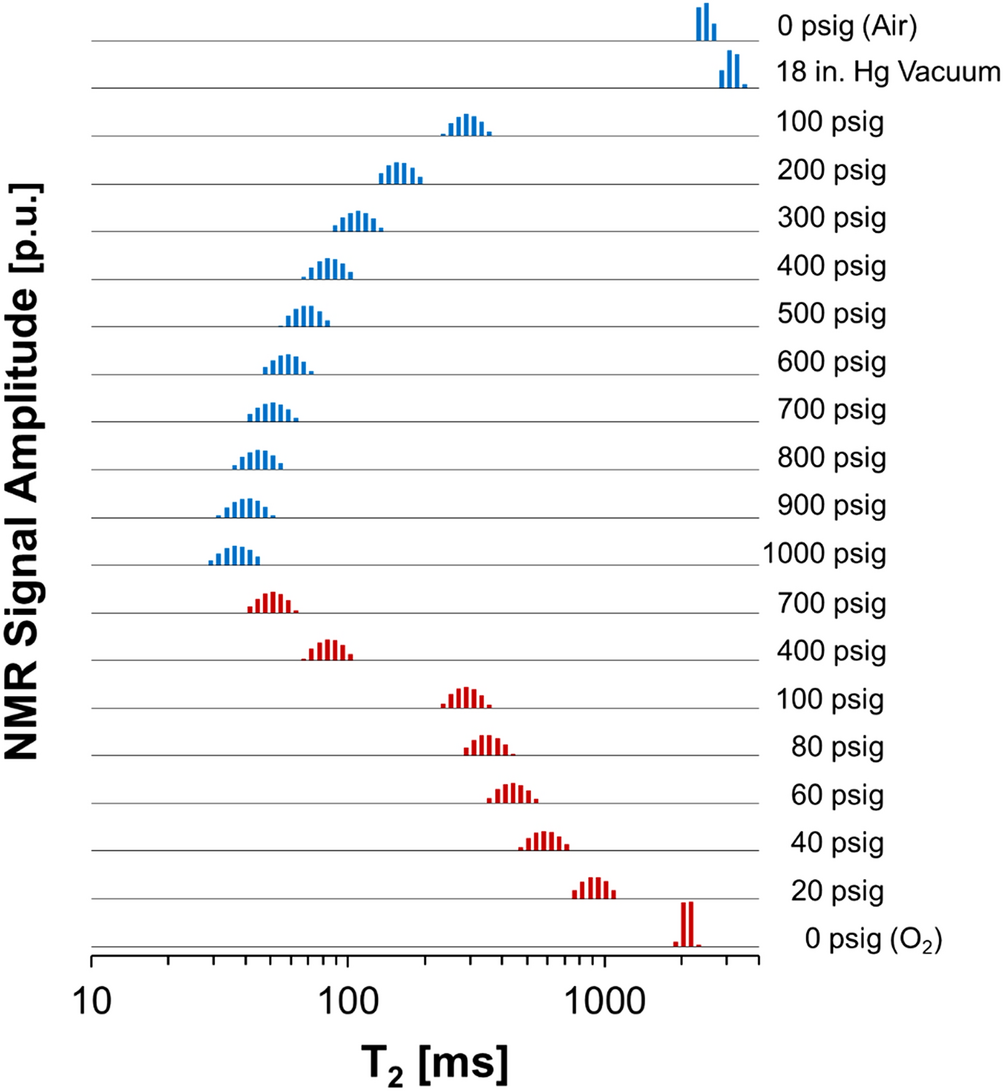
Quantification of dissolved O2 in bulk aqueous solutions and porous media using NMR relaxometry | Scientific Reports
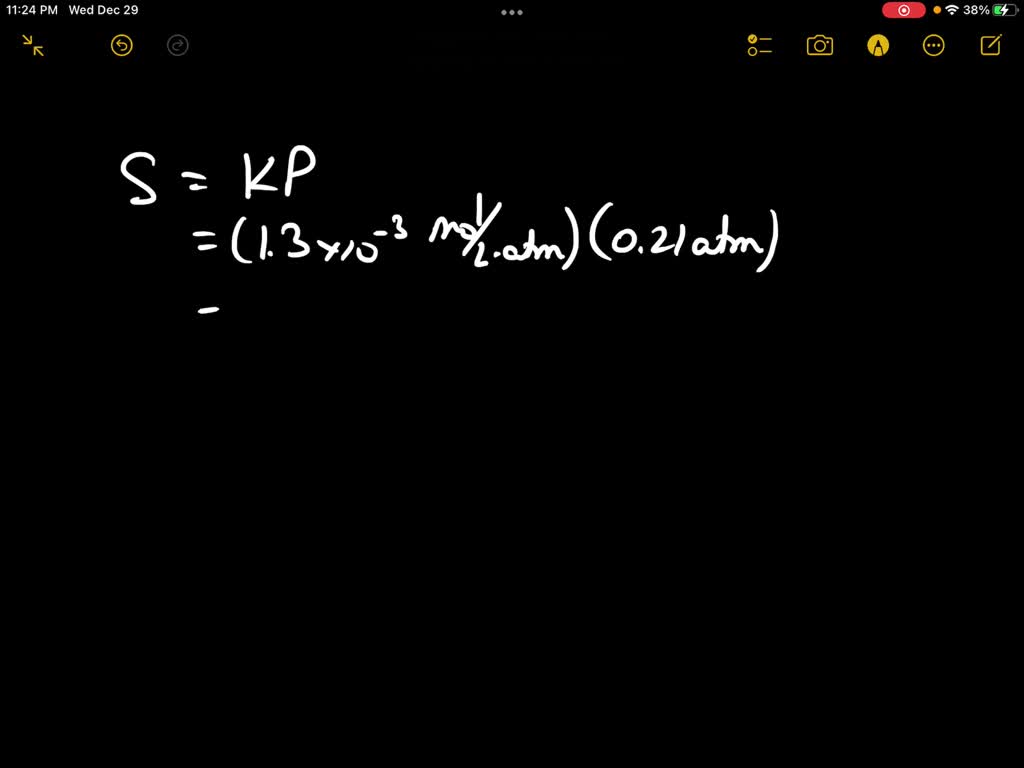
SOLVED: Enter your answer in the provided box. Calculate the molar concentration of oxygen in water at 25 ° C for a partial pressure of 0.21 atm. The Henry's law constant for

What is the concentration of O_2(g) in water at 25 degree C exposed to a partial pressure of oxygen of 325 mmHg? The Henry's law constant for oxygen gas at 25 degree

The Henry's law constant for oxygen gas in water at `25^(@)C` is `1.3xx10^(-3)` M `atm^(-1)`. - YouTube
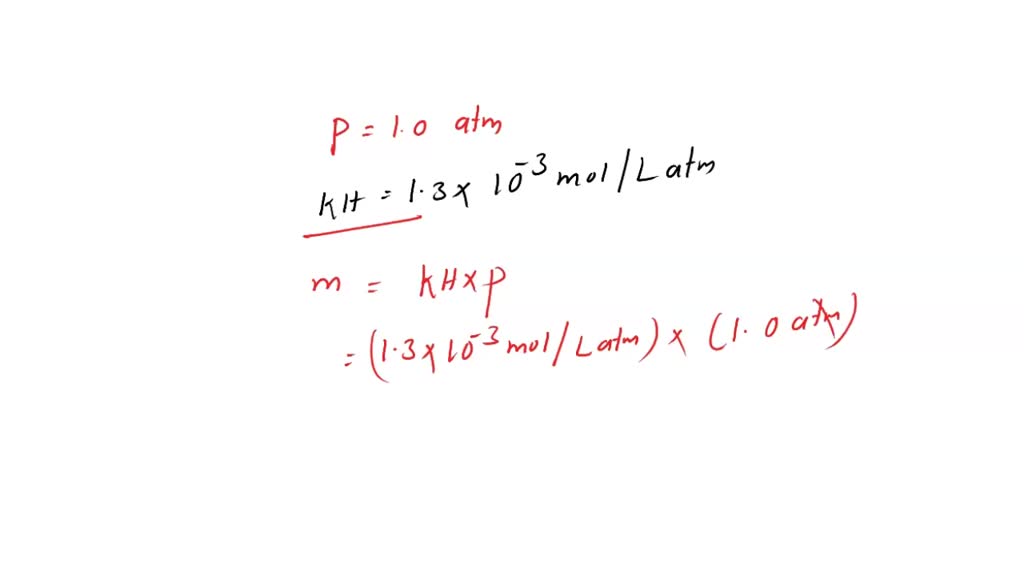
SOLVED: What is the molar concentration of oxygen in water at 25C for a partial pressure of 1.0 atm (KH = 1.3 x 10-3 mol/Latm)

SOLVED: Enter your answer in the provided box: Calculate the molar concentration of oxygen in water at 258C for a partial pressure of 0.27 atm: The Henry's law constant for oxygen is

SOLVED: Calculate the molar concentration of oxygen in water at 25°C for a partial pressure of 0.24 atm. The Henry's law constant for oxygen is 1.3 × 10−3 mol/L · atm. Report

What is the molar concentration of a 6.8*g mass of hydrogen peroxide, that is dissolved in a volume of 100*mL of water? | Socratic

SOLVED: Calculate the molar concentration of oxygen in water at 25°C for a partial pressure of 0.24 atm. The Henry's law constant for oxygen is 1.3 × 10−3 mol/L · atm. Report