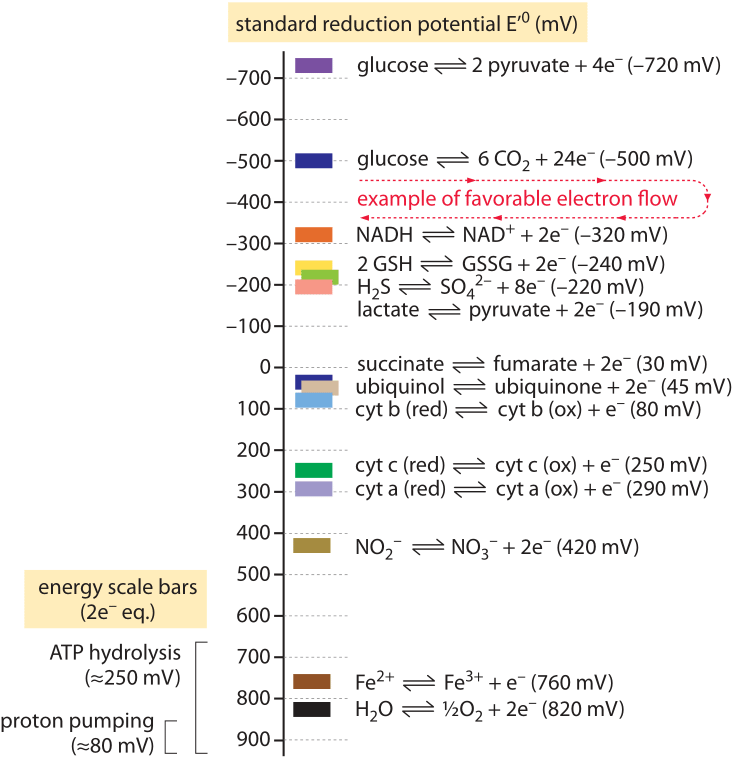
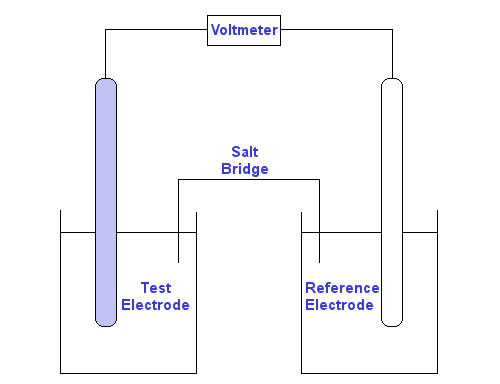
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry

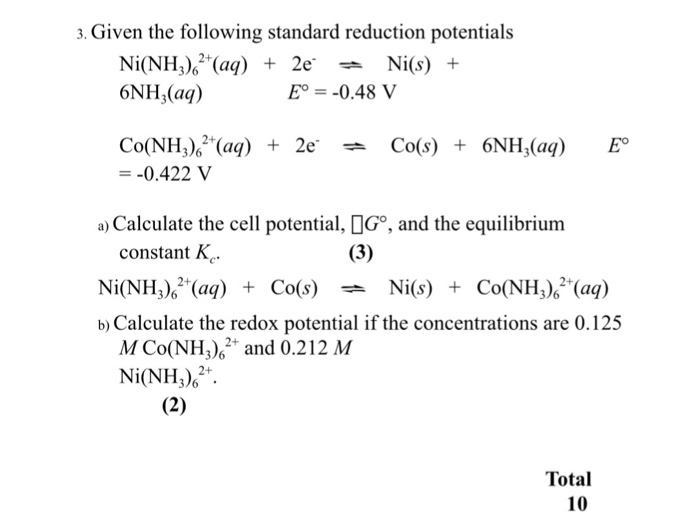
Given standard electrode potentials: Fe^3 + + 3e^-→ Fe;E^0 = - 0.036V Fe^2 + + 2e^-→ Fe;E^0 = - 0.440V The standard electrode potential E^o for Fe^3 + + e^ - → Fe^2 + is:

Absolute Potential of the Standard Hydrogen Electrode and the Problem of Interconversion of Potentials in Different Solvents | The Journal of Physical Chemistry B
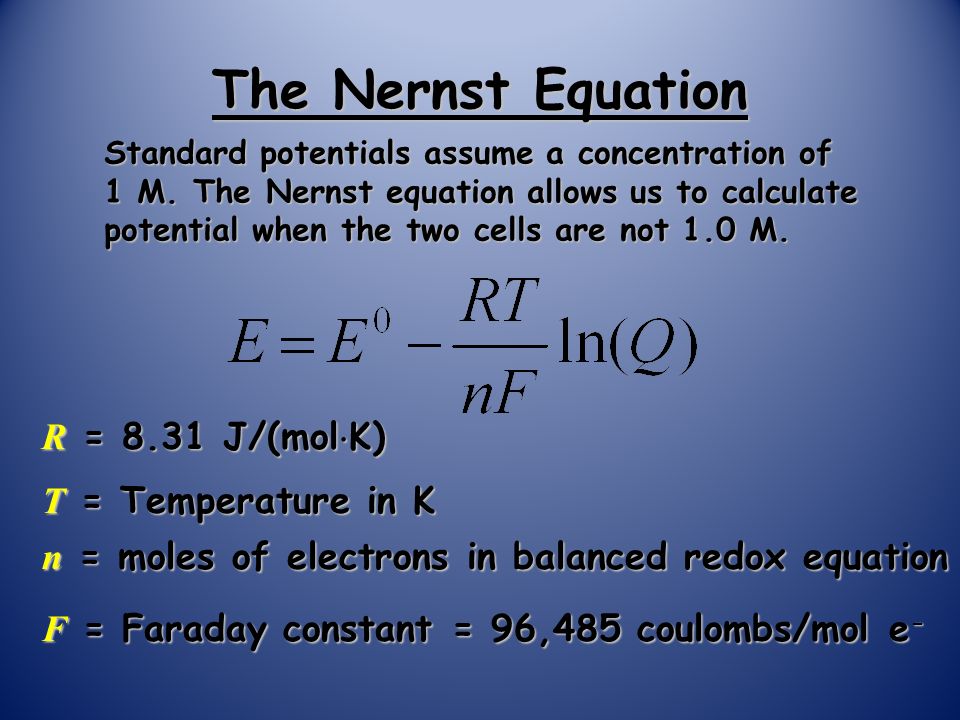
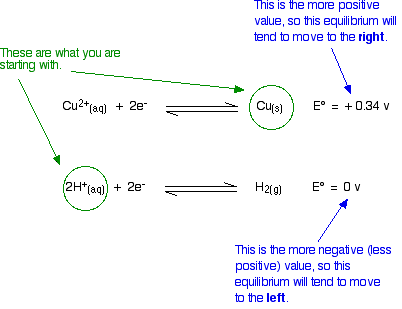
The Nernst Equation Standard potentials assume a concentration of 1 M. The Nernst equation allows us to calculate potential when the two cells are not. - ppt download

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa
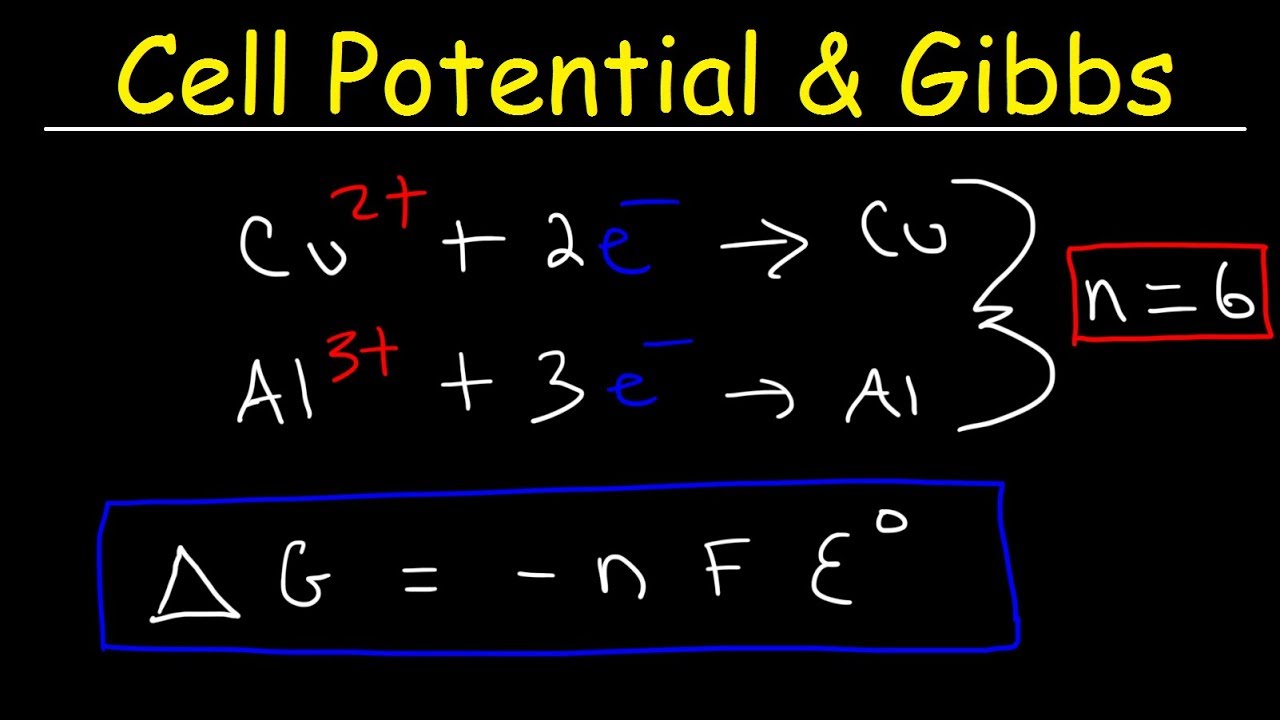
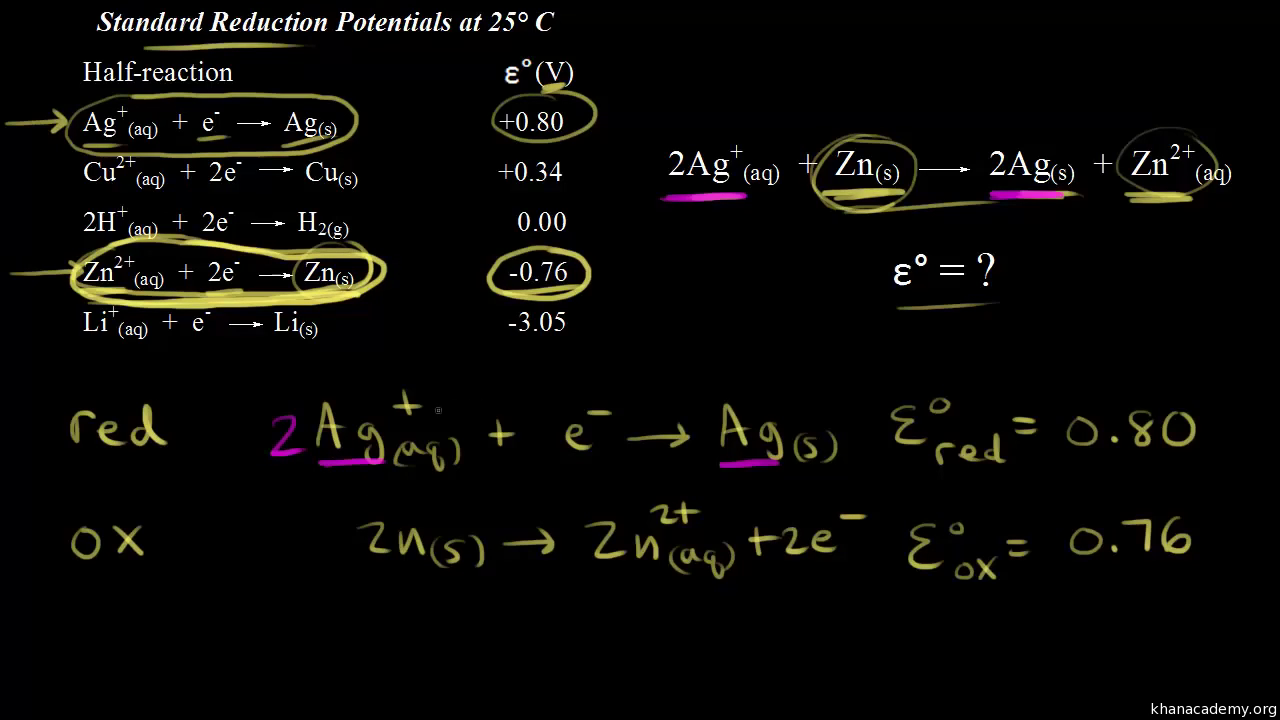
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems - YouTube




:max_bytes(150000):strip_icc()/Standardreductionpotential-5b551731c9e77c003ec223b3.jpg)