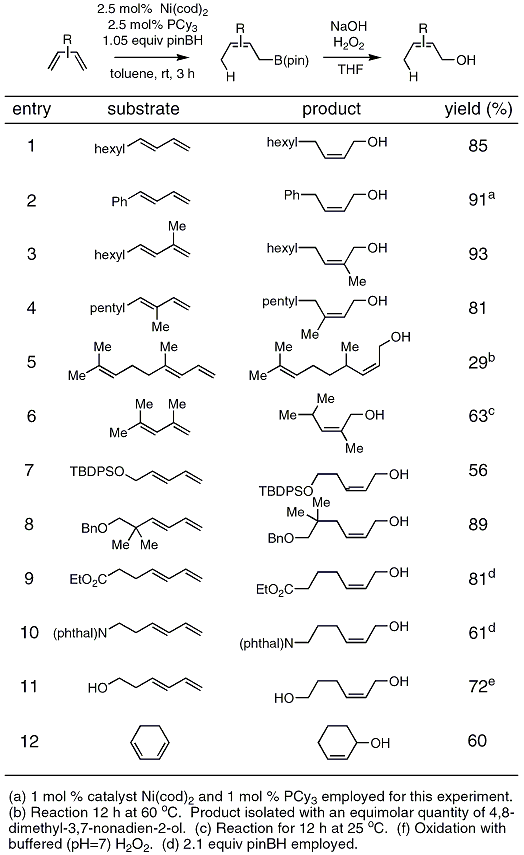
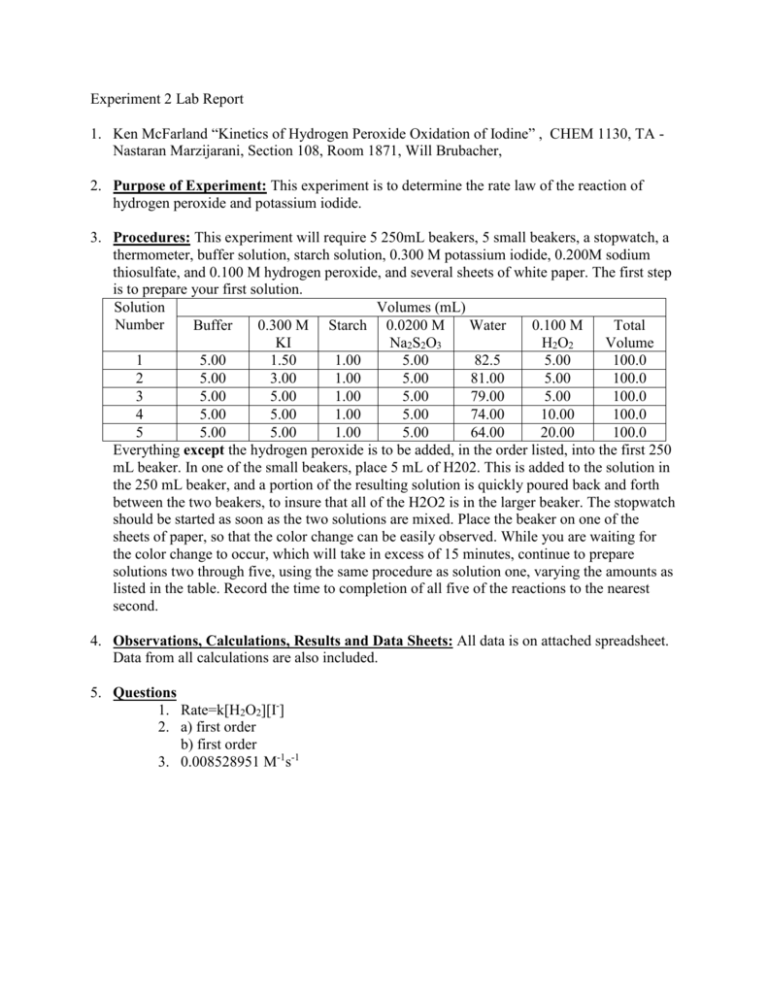
Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry
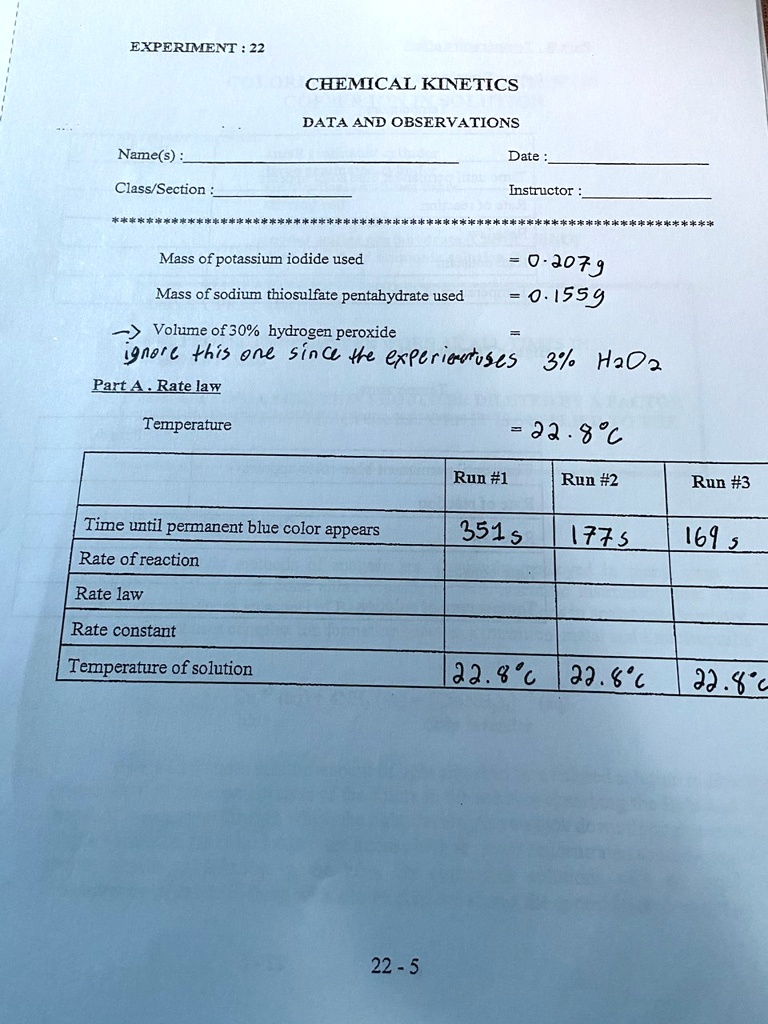
SOLVED: EXPERIMENT CHEMICAL KINETICS DATA AND OBSERVATIONS Name(s) Date Class/Section Instructor #450860a Mass of potassium iodide used 0- J079 0. 1559 Mass of sodium thiosulfate pentahydrate used Volume of 30% hydrogen peroxide
The curve shown below shows the variation of time against temperature for the reaction between - Tutorke
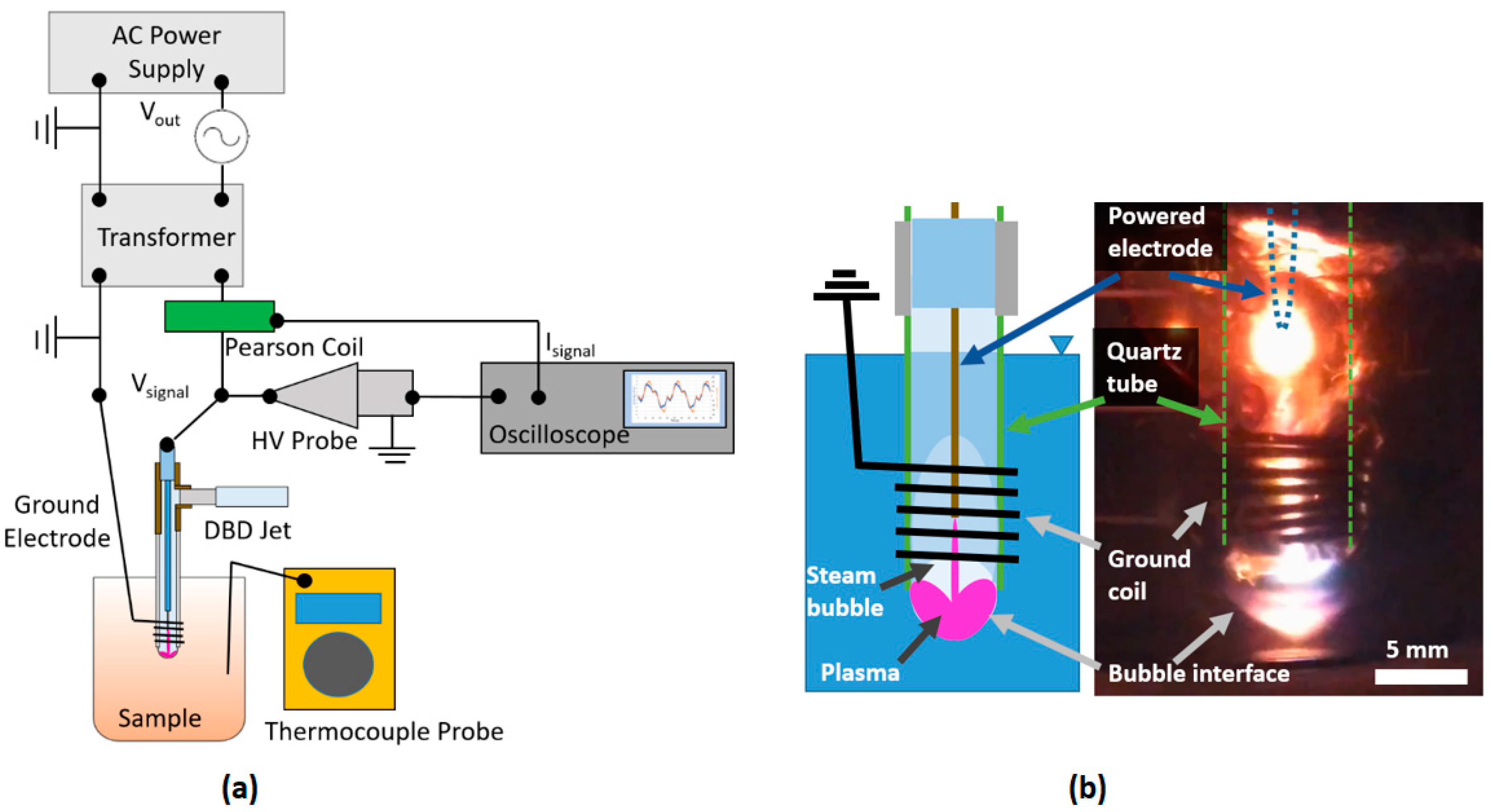
Plasma | Free Full-Text | Hydrogen Peroxide Interference in Chemical Oxygen Demand Assessments of Plasma Treated Waters
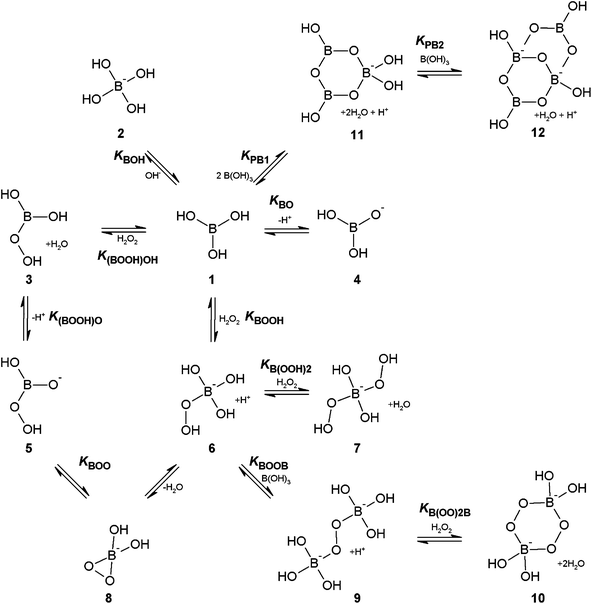
A kinetic and theoretical study of the borate catalysed reactions of hydrogen peroxide : the role of dioxaborirane as the catalytic intermediate for a ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB26842F

Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium(III) - ScienceDirect

Rate of Decomposition of Hydrogen Peroxide (1.4.1) | OCR A Level Chemistry Revision Notes 2017 | Save My Exams
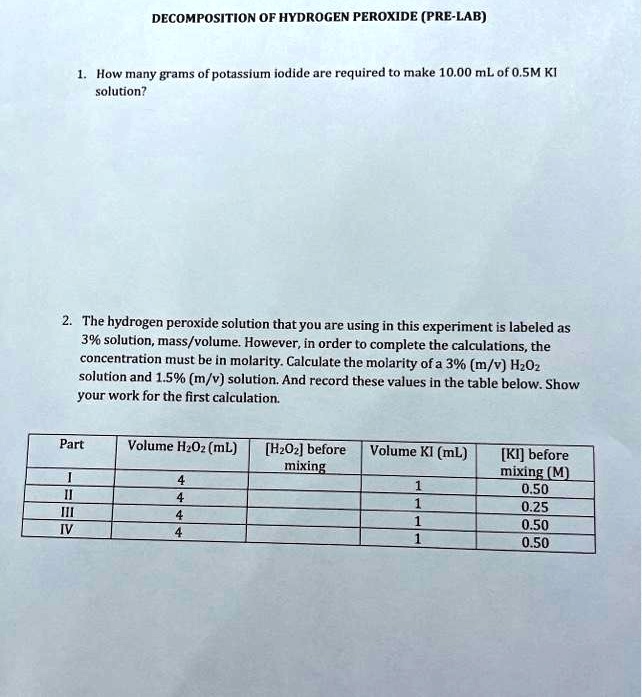
SOLVED: DECOMPOSITION OF HYDROGEN PEROXIDE (PRE-LAB) How many grams of potassium iodide are required to make 10.00 mLofO5M KI solution? The hydrogen peroxide solution thatyou are using in this experiment is labeled

Rate of Decomposition of Hydrogen Peroxide (1.4.1) | OCR A Level Chemistry Revision Notes 2017 | Save My Exams

Rapid reaction of sulfide with hydrogen peroxide and formation of different final products by freezing compared to those in solution - Takenaka - 2003 - International Journal of Chemical Kinetics - Wiley Online Library
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide Molecular equation: 2KI(aq) + 2HCl(aq) + H O (aq) I (s) +