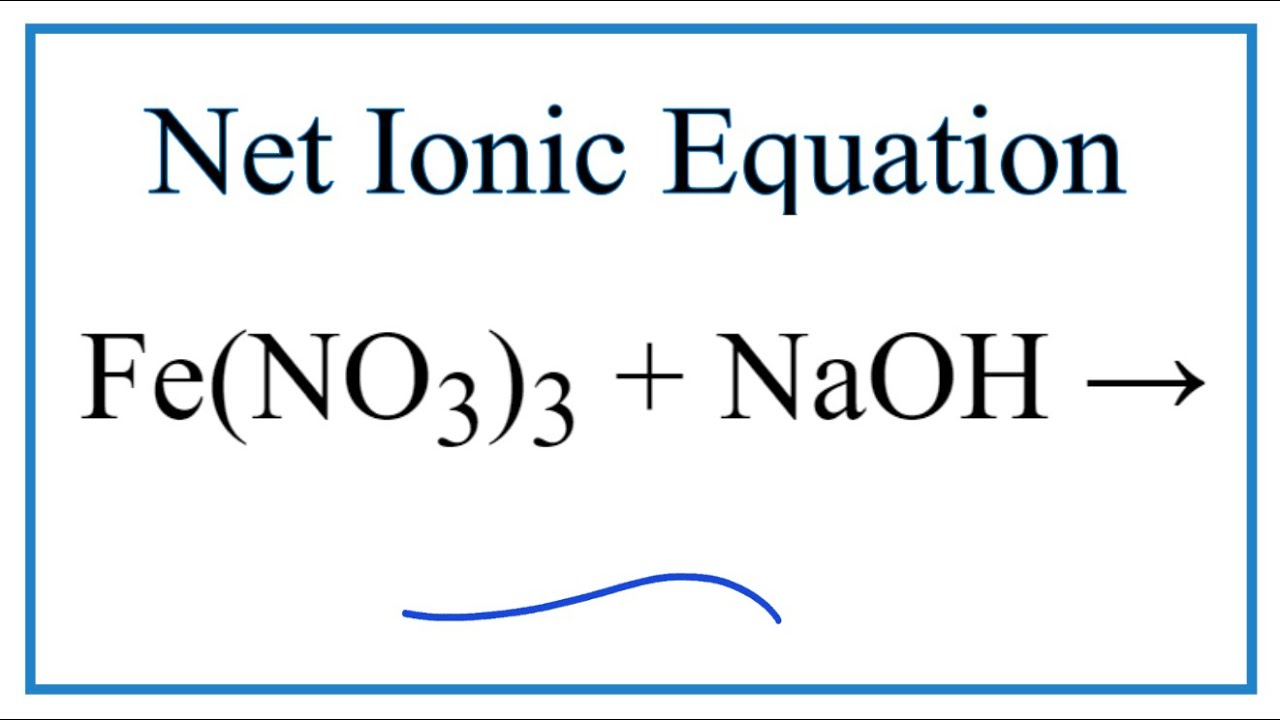Photo-catalytic selectivity of anthranilic acid over iron oxide incorporated titania nanoparticles: Influence of the Fe2+/Fe3+ ratio of iron oxide - ScienceDirect

Iron transition metal Chemistry iron(II) Fe2+ iron(III) Fe3+ complexes ions ligand substitution redox chemical reactions principal oxidation states +2 +3 extraction GCE AS A2 IB A level inorganic chemistry revision notes
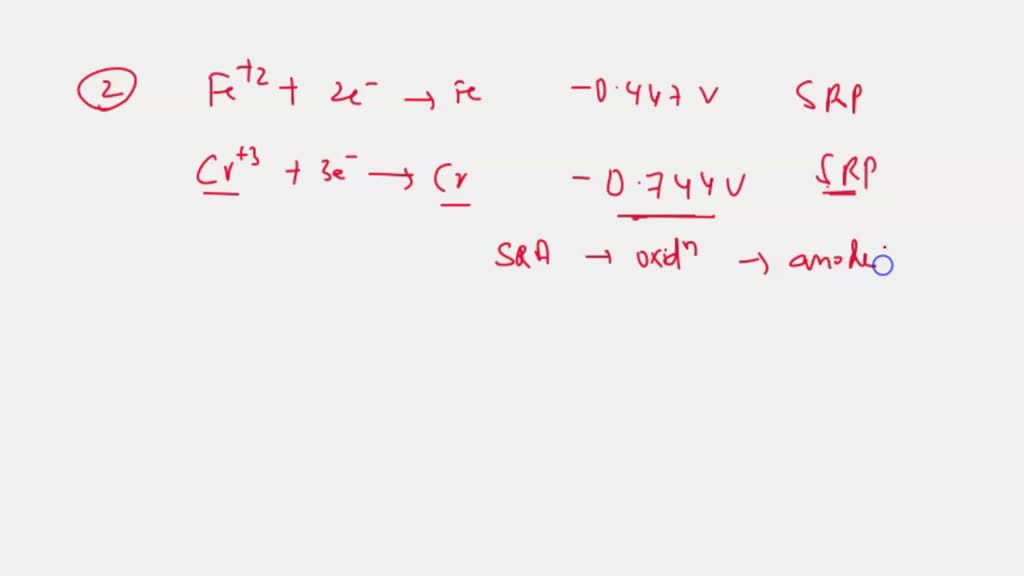
SOLVED: Iron(III) was reduced to iron(II) by chromium(III) in acidic solution according to the following unbalanced reaction: Cr3+(aq) + Fe3+(aq) ⟷ Fe2+(aq) + chromium oxide compound(aq) The experiment requires 66.9 g of

Figure 3 from First-Principles Fe L2,3-Edge and O K-Edge XANES and XMCD Spectra for Iron Oxides. | Semantic Scholar

Gold, Silver, and Iron Oxide Nanoparticle Incorporation into Silk Hydrogels for Biomedical Applications: Elaboration, Structure, and Properties | ACS Biomaterials Science & Engineering

Chemical Origin of Sodium Phosphate Interactions on Iron and Iron Oxide Surfaces by First Principle Calculations | The Journal of Physical Chemistry C

PDF) Phase Transformation of Iron Oxide Nanoparticles by Varying the Molar Ratio of Fe2+: Fe3+ | Samaneh Alibeigi - Academia.edu

Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides | Nature

Iron oxide (Fe0.94O) exist. What reaction of iron ions i.e. Fe^2 + and Fe^3 + present in this? Calculate their percentage.

Electrochemical Analysis of Changes in Iron Oxide Reducibility during Abiotic Ferrihydrite Transformation into Goethite and Magnetite | Environmental Science & Technology

Determining Magnetite/Maghemite Composition and Core–Shell Nanostructure from Magnetization Curve for Iron Oxide Nanoparticles | The Journal of Physical Chemistry C

Figure 1 from Electronic spectra of Fe 3 + oxides and oxide hydroxides in the near IR to near UV | Semantic Scholar

Iron transition metal Chemistry iron(II) Fe2+ iron(III) Fe3+ complexes ions ligand substitution redox chemical reactions principal oxidation states +2 +3 extraction GCE AS A2 IB A level inorganic chemistry revision notes

Droop, G. T. R. (1987) A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineralogical Magazine, 51 (361). 431-435 doi:10.1180/minmag.1987.051.361.10

The Estimation of Iron (II) and Iron (III) in a Mixture Containing Both - Free comparison essay example, compare and contrast paper

![A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0304885317309411-gr8.jpg)


![A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0304885317309411-gr4.jpg)
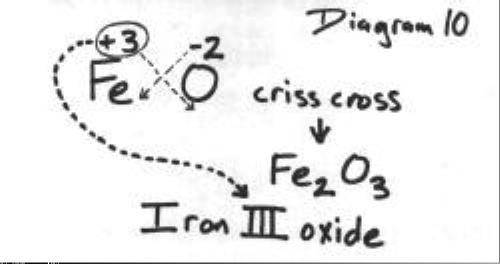


![Solved 3. Gravimetric Determination of Iron as Fe2O3] A | Chegg.com Solved 3. Gravimetric Determination of Iron as Fe2O3] A | Chegg.com](https://media.cheggcdn.com/media/5bc/5bc6b959-5764-4171-9b45-b3e05ae71aa6/php5tXDO9)